Counterfeit Ozempic products surfaced in Germany’s official supply chain in October 2023, as reported by Medscape Medical News. The World Health Organization (WHO) recently reported on three additional counterfeit batches of Ozempic that were discovered in late 2023 in Brazil, the United Kingdom, and the United States, with an unknown number of unreported cases.
Novo Nordisk, the manufacturer, has confirmed that the batches in question are not original products. The pens may be ineffective or contain harmful ingredients. Toxicology results are currently unknown. Such counterfeit products easily enter additional markets through drug importers or reimporters.
Supply Shortages
Semaglutide is approved for the treatment of type 2 diabetes (Ozempic) or obesity (Wegovy), and Ozempic is used off-label for obesity. Given the market situation, it is not surprising that such products have become a target for counterfeiters.
“The sustained high demand for Ozempic exceeds production capacities,” wrote Novo Nordisc. “To ensure the supply of Ozempic 1 mg, particularly for individuals already being treated with Ozempic for type 2 diabetes, the focus is on providing Ozempic 1 mg in agreement with the relevant authorities.”
Therefore, in the second quarter of 2024, the introductory dosage of Ozempic 0.25 mg will not be delivered, and Ozempic 0.5 mg will be delivered with restrictions. In other words, the product cannot be initiated in new patients. To reduce side effects, an initial subcutaneous dose of 0.5 mg is administered weekly and eventually increased to 1 mg weekly.
Recognizing Counterfeit Products
Yukiko Nakatani, MD, PhD, WHO assistant director general for access to medicines and health products, advises physicians and pharmacists to be particularly vigilant with these products. The WHO has identified the following characteristics of counterfeit packages:
- The batch number LP6F832 does not exist.
- The combination of batch number NAR0074 with serial number 430834149057 does not match manufacturing records.
- The batch number MP5E511 is correct, but the product is counterfeit.
Pharmacists in all countries should pay attention to the following criteria when dispensing medication:
- The WHO advises against dispensing products labeled with suspicious batch numbers listed online.
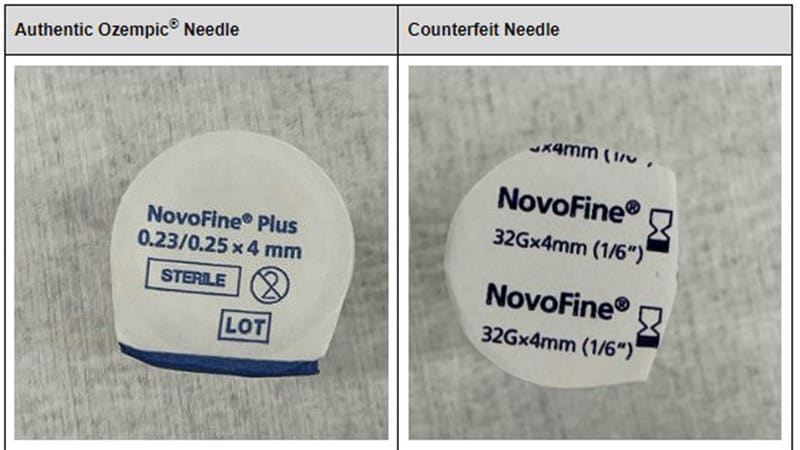
- Counterfeit Ozempic pens, unlike the originals, may have a scale protruding from the pen when the user adjusts the dose.
- The label may not adhere well or at all to the pen.
- Spelling errors may be present on the front of the box.
Increased Security in Germany
In Germany, the Federal Institute for Drugs and Medical Devices (BfArM) is responsible for secure pharmacotherapy. “In addition to the known counterfeit of the drug Ozempic 1 mg (product code: 04150153985573, serial number: 1946483405690, and batch: MP5E511), no further packets with the counterfeit serial number or counterfeits with other serial numbers have been identified,” a statement reads.
The BfArM nevertheless advises pharmacists to open the secondary packaging before dispensing the drug and to check the primary packaging for authenticity. The boxes often appear genuine, but there are differences in the pens.
In addition, in Germany, the securPharm system is available as another effective tool to detect counterfeit drugs. It was developed in accordance with the European Union (EU) Falsified Medicines Directive (2011/62/EU).
Each drug package receives a unique serial number, product code, batch number, and expiration date in the form of a Data Matrix code on the packaging before delivery from the factory. Manufacturers upload the serial numbers and other product information to a central database managed by securPharm. Throughout the supply chain (eg, at wholesalers or pharmacies), employees scan the package and verify the code. If the serial number has already been used or does not exist in the database, the software issues an alert, and the medication cannot be dispensed.
In addition to the Data Matrix code, the packaging features tamper-evident seals to ensure the packaging has not been opened unnoticed.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.